Rethinking Protein Modifications in Reproductive Biology
The latest studies on protein modifications have sparked a fascinating discussion in the scientific community. In particular, the research on lactylation in porcine granulosa cells is generating buzz. Traditionally, lactate was seen merely as a byproduct of metabolism, but recent work has revealed that this molecule may have a much more active role. This opinion piece takes a closer look at these findings, examines their real-world implications, and offers a neat perspective on how they might reshape our understanding of reproductive physiology.
At the core of the discussion is the observation that lactate can attach to proteins – a process referred to as lactylation. Far from being a waste product, lactate now appears to contribute to the regulation of protein activities in cells. As we take a closer look at this emerging field, we begin to understand that these modifications might be key to the natural regulation of ovarian functions, thus affecting hormone production, oocyte maturation, and overall fertility.
Exploring Lactate’s Active Role in Cellular Regulation
For decades, cellular metabolism was thought to focus primarily on energy production and the disposal of byproducts. However, the discovery that lactate engages in post-translational modifications – the process of altering proteins after they are made – has forced scientists to rethink this role. Researchers have now uncovered that lactate can attach to lysine residues on proteins, a process similar to more commonly known modifications like phosphorylation or acetylation.
This shift in thinking matters for several reasons. First, it challenges the simplistic view of lactate as merely a metabolic leftover. Instead, lactate changes the very dynamic of cellular signaling, influencing everything from gene expression to energy metabolism. When we dig into the topic, we see that the cellular environment is full of tricky parts and tangled issues, where even small distinctions in protein structure and function can have enormous downstream effects.
Modern Techniques for Mapping the Lactylome
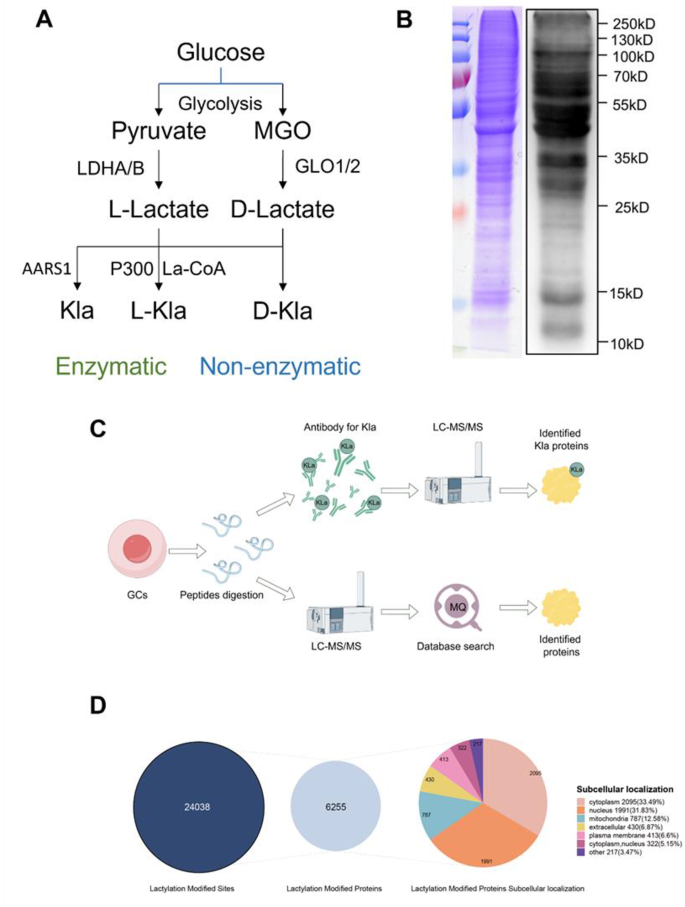
One of the highlights of the recent study is the use of advanced mass spectrometry. This technique provides detailed insights into the little details of protein modifications. With mass spectrometry, researchers are able to accurately profile all proteins that undergo lactylation in granulosa cells. This approach not only identifies lactylated proteins but also quantifies the extent of modification on each one.
Below is a summary of the key techniques used, organized in both list and table format:
- High-resolution mass spectrometry for precise measurement
- Protein isolation and enrichment strategies
- Bioinformatics analysis to map the modified sites across the proteome
- Comparative studies between different cellular conditions
| Technique | Description |
|---|---|
| Mass Spectrometry | Detects and quantifies lactylated peptides with high precision |
| Protein Enrichment | Isolates lactylated proteins from the complex mixture found in cells |
| Bioinformatics | Analyzes mass spectrometry data to map modification sites |
These methods offer a detailed snapshot of the lactylome, revealing subtle parts of protein regulation that were previously hidden. The integration of cutting-edge technology with conventional biochemical techniques is not only innovative but also opens the door to a deeper understanding of cellular function.
Unpacking the Fertility Implications of Lactylation
Granulosa cells are essential for the maturation of oocytes, and they are crucial players in hormone production. Recent findings suggest that lactylation might regulate key functional roles in these cells. This revelation provides an intriguing link between metabolism and reproductive health, stirring up thought-provoking discussions among researchers and clinicians alike.
When we poke around the subject, several important questions arise:
- Could lactate-induced modifications affect the rate at which oocytes mature?
- Do these modifications play a role in the hormonal shifts observed during the menstrual cycle?
- How do metabolic conditions, such as obesity or diabetes, influence lactylation patterns?
Such inquiries are critical because they not only enhance our understanding of ovarian biology but also hint at potential therapeutic strategies. For instance, if lactate plays a regulatory role in fertility, then targeting lactylation pathways may provide ways to improve or restore ovarian function in individuals facing fertility challenges.
Linking Metabolic Health and Reproductive Physiology
A fascinating aspect of this research is the evident connection between metabolic health and reproductive capacity. Cells must manage a host of confusing bits and tangled issues when it comes to metabolism, and lactate appears to be right at the center of this struggle. The latest insights show that nutrient status and metabolic shifts can directly influence protein functions through lactylation.
Here are some points to consider that illustrate this metabolic-reproductive interplay:
- Energy Metabolism Impact: Altered energy states may change the extent of lactylation and, consequently, how granulosa cells handle oocyte development.
- Hormonal Regulation: Variations in lactate levels could influence hormone output in the ovary, thereby affecting fertility.
- Stress Response: Lactate might help cells manage stress by modifying proteins that deal with oxidative stress or other challenges.
These connections underscore a critical point: metabolic changes are not isolated events. They ripple through a network of protein modifications that can ultimately control cellular behavior in profound ways. By staying curious and taking a closer look at this interplay, researchers can piece together a narrative that bridges energy production and reproductive health.
Looking at the Tricky Parts of Lactylation Research
As with all emerging fields, there are several tricky parts to consider. The study of lactylation is still in its early days, and several complicated pieces of the puzzle remain unsolved. The current research provides a good foundation but also highlights the need for further exploration into the subtle parts of how lactate interacts with other cellular components.
Some of the challenges include:
- Identifying Affected Proteins: With thousands of proteins within a cell, pinpointing which ones are consistently lactylated is a nerve-racking task.
- Understanding Functional Outcomes: Even after a protein is modified, understanding its new role can be like navigating a maze full of twists and turns.
- Technical Limitations: Despite advances in mass spectrometry, detecting low-abundance modifications remains a daunting challenge.
- Physiological Diversity: Differences between species and between cell types mean that findings in porcine cells might not fully translate to humans.
While these challenges are off-putting, they also serve as a motivator for the scientific community to break new ground. With every new study, researchers are better equipped to figure a path through these confusing bits and bring us closer to practical applications.
Comparing Lactylation with Other Protein Modifications
It’s useful to compare lactylation with other well-known protein modifications. For many years, scientists have focused on phosphorylation and acetylation as key regulators of protein function. The arrival of lactylation into the spotlight raises interesting questions about how these processes overlap and interact.
The following table outlines some of the main distinctions between these modifications:
| Aspect | Lactylation | Phosphorylation/Acetylation |
|---|---|---|
| Source Molecule | Lactate (metabolic byproduct) | Adenosine triphosphate (ATP), Acetyl-CoA |
| Role in Signaling | May modulate metabolic enzymes and regulatory proteins | Widely involved in signaling cascades and enzyme regulation |
| Research Maturity | Emerging field with many twists and turns | Well established with a long history of study |
This comparison reveals that while phosphorylation and acetylation are already celebrated as key modulators, lactylation could offer a new layer of control. With further research, these modifications may be seen as complementary systems that together fine-tune cellular behavior.
The Broader Impact of Lactate Dynamics on Cellular Health
The discovery of lactylation has broader implications that extend beyond reproductive biology and into our general understanding of cellular health. The idea that a simple molecule like lactate can be repurposed to affect protein function is both surprising and exciting.
Some of the broader takeaways include:
- Reinterpreting Waste: Metabolites, once thought to be dead-end products, might actually carry essential signals. Recognizing these signals can help us reframe our approach to other metabolic byproducts.
- Dynamic Regulation: Cells constantly adjust to their environment. Lactylation is one of the many ways that cells might respond to changes in nutrient availability or stress levels.
- Therapeutic Opportunities: If we can tweak how lactate modifies proteins, there may soon be treatments for various conditions ranging from fertility issues to metabolic disorders.
This fresh perspective is driving conversations not just in the lab, but also in clinical settings where personalized treatments may be developed by harnessing these new understandings. In the near future, clinicians might be able to design interventions that steer cellular behavior by modulating these subtle details in protein activity.
Addressing the Off-Beat Challenges in Experimental Design
Beyond the scientific breakthroughs, the path to understanding lactylation is full of experimental challenges. Researchers must work through many confusing bits when setting up experiments to isolate and analyze lactylated proteins. Some of these challenges include:
- Ensuring Specificity: It is critical to make sure that the modifications detected are truly due to lactate attachment and not other similar small molecules.
- Establishing Baselines: Given that metabolic states may vary considerably among different cells, control experiments must be rigorously designed.
- Overcoming Technical Hurdles: The tools involved, such as high-resolution mass spectrometers, are expensive and require specialized handling, adding an intimidating layer to the research process.
Such challenges may be nerve-racking, but they are also opportunities for refining methodologies. Each new study paves the way for better protocols and deeper insights, eventually brightening the future of lactylation research.
Future Directions: Opportunities and Potential Therapies
The potential for lactylation research to influence reproductive health and beyond is super important. As scientists get into this field, new questions emerge that could lead to innovative therapies. Some possibilities include:
- Fertility Enhancement: By regulating the lactylation of key proteins, it might be possible to directly improve oocyte quality and maturation, offering a new angle on fertility treatments.
- Metabolic Disorder Management: Since lactate dynamics are central to overall cellular metabolism, interventions targeting lactylation could be explored as treatments for conditions like type 2 diabetes and obesity.
- Stress and Aging: Understanding how lactate influences protein response to oxidative stress may lead to strategies that delay the effects of aging at the cellular level.
Moreover, a better grasp of lactylation can help develop diagnostic markers. For example, variations in protein lactylation profiles might one day serve as indicators for ovarian reserve or metabolic imbalances, allowing clinicians to customize treatments for individual patients.
Charting a Course Through the Twists and Turns of Cellular Metabolism
With each new discovery, the field of metabolic research grows richer and more intricate. While the idea of lactate acting as a signaling molecule might have sounded far-fetched only a few years ago, it is now emerging as a key piece in the puzzle of cellular regulation. Overcoming the maze of tricky parts and tangled issues in protein modification research is not easy, but it is an effort that holds a lot of promise.
As the underlying studies continue to take shape, we can expect further exploration of how proteins are modified by lactate in various types of cells – not just those found in the ovarian follicle. This research offers a tantalizing glimpse into the hidden complexities of cell biology, urging us all to take a closer look at the often-overlooked elements of metabolic regulation.
Integrating Research Findings with Real-World Applications
The ripple effects of these scientific advancements do not stop at academic interest. There is a growing demand for bridging the gap between basic research and its implications for daily living. For homeowners, patients, and everyday individuals, improved understanding of cell metabolism can pave the way for new treatments that enhance quality of life.
Consider these broader benefits:
- Personalized Medicine: As researchers unlock the secrets of lactylation, targeted therapies may become available that treat reproductive and metabolic issues on an individual basis.
- Preventative Approaches: Early detection markers based on protein modifications could alert physicians to developmental or metabolic imbalances before they become serious.
- Holistic Health Strategies: A better understanding of how metabolic byproducts affect cellular function could encourage lifestyle changes or dietary interventions that help maintain overall health.
The evolution of this research not only opens a new chapter in the life sciences but can also inform public health policies. As more data is collected, integrating these findings into the realm of medical practice could lead to the development of holistic strategies that address both metabolic and reproductive challenges concurrently.
Looking Ahead: A Future Fueled by Metabolic Insights
In conclusion, the emerging research on lactylation in porcine granulosa cells offers a refreshing lens through which we can view cellular metabolism and reproductive biology. The exciting discoveries in this field remind us that there is still much to learn about the ways small molecules like lactate influence our body’s systems.
When we step back and assess the broader picture, it becomes evident that the relationship between metabolism and reproductive function is loaded with potential. Although the road ahead is full of tricky parts and nerve-racking challenges, the promise of new therapeutic avenues and diagnostic techniques makes these research endeavors not only interesting but also potentially transformative.
As this area of study matures, one can only anticipate that future investigations will continue to uncover subtle details, provide deeper insights, and ultimately translate these findings into clinical applications that benefit society at large. By staying open to fresh perspectives and embracing the hidden complexities of cellular regulation, we can look forward to an era where metabolic insights lead directly to improved reproductive health and overall well-being.
Final Thoughts: Embracing New Paradigms in Cellular Research
The journey into understanding lactylation is just beginning, and the path is dotted with challenging bits, tangled issues, and nerve-racking moments. Yet, every step taken in this line of research brings us closer to a more nuanced view of how our cells function and respond to their environment.
For those in the home improvement arenas of scientific research and biomedical practice, these developments serve as a reminder that innovation often comes from rethinking old assumptions. Holding space for new ideas—especially those that recast waste products as essential cellular messengers—can energize entire fields and lead to breakthroughs that improve lives.
Moving forward, collaboration between biochemists, clinicians, and even experts in technological applications will be key to steering through these complicated pieces of cellular regulation. With the promise of advanced tools and integrated research strategies, the future of lactylation studies is both exciting and promising.
By taking a closer look at these dynamic processes, we gain a better understanding of how metabolism, protein modifications, and reproductive health are intricately linked. Ultimately, this fresh perspective not only enriches our scientific boundaries but also holds the promise of novel, effective therapies that could one day enhance human well-being in ways we are only beginning to imagine.
Originally Post From https://bioengineer.org/mapping-the-lactylome-in-porcine-granulosa-cells/
Read more about this topic at
Decoding Osteosarcoma’s Lactylation Gene Expression
Histone lactylation dynamics: Unlocking the triad of …